MRI Contrast Reaction Management: Documentation & Protocol Explained


Key Takeaways
- Thorough documentation of all contrast reactions, regardless of severity, is required for legal protection, future care planning, and regulatory compliance.
- Standardized Electronic Health Record (EHR) templates with mandatory fields for contrast details, reaction classification, interventions, and timestamps reduce cognitive load during emergencies and ensure consistent documentation.
- Clear staff role assignments, including a team leader, medication administrator, documentation specialist, and resource coordinator, should be established before emergencies occur through shift huddles and regular simulation drills.
- Post-reaction monitoring protocols must be proportional to severity, with specific discharge criteria including stable vital signs, reliable transportation, and written instructions for warning signs.
- ContrastConnect offers a remote radiologist supervision model for facilities facing staffing challenges, providing real-time support during contrast exams and reactions, along with audit-ready documentation for regulatory compliance.
Identifying & Managing MRI Contrast Reactions
Contrast reactions typically manifest within minutes of administration, with most acute reactions occurring within the first hour. MRI contrast reactions fall into two broad categories: allergic-like and physiologic, with severity classifications of mild, moderate, and severe determining the appropriate response pathway.
Mild Reaction Symptoms
Mild reactions rarely require medical intervention beyond monitoring and reassurance. These self-limiting reactions include scattered hives, pruritus, mild localized edema, transient nausea, brief vomiting, or mild dizziness. Documentation is required, but emergency medication administration and extended observation are generally unnecessary.
Moderate Reaction Symptoms
Moderate reactions require prompt medical intervention and close monitoring. These include diffuse urticaria, facial edema without respiratory distress, mild bronchospasm, mild to moderate hypotension responsive to fluids, symptomatic tachycardia or bradycardia, persistent vomiting, or pronounced dizziness.
Severe Reaction Warning Signs
Severe reactions constitute medical emergencies that require immediate action and the activation of emergency response protocols. These life-threatening reactions include respiratory arrest, cardiac arrhythmias, seizures, progressive angioedema affecting the airway, and hemodynamic instability with hypotension unresponsive to initial fluid challenges. Facilities must maintain emergency equipment and staff trained in advanced life support.
The Essential Response Protocol for MRI Contrast Reactions
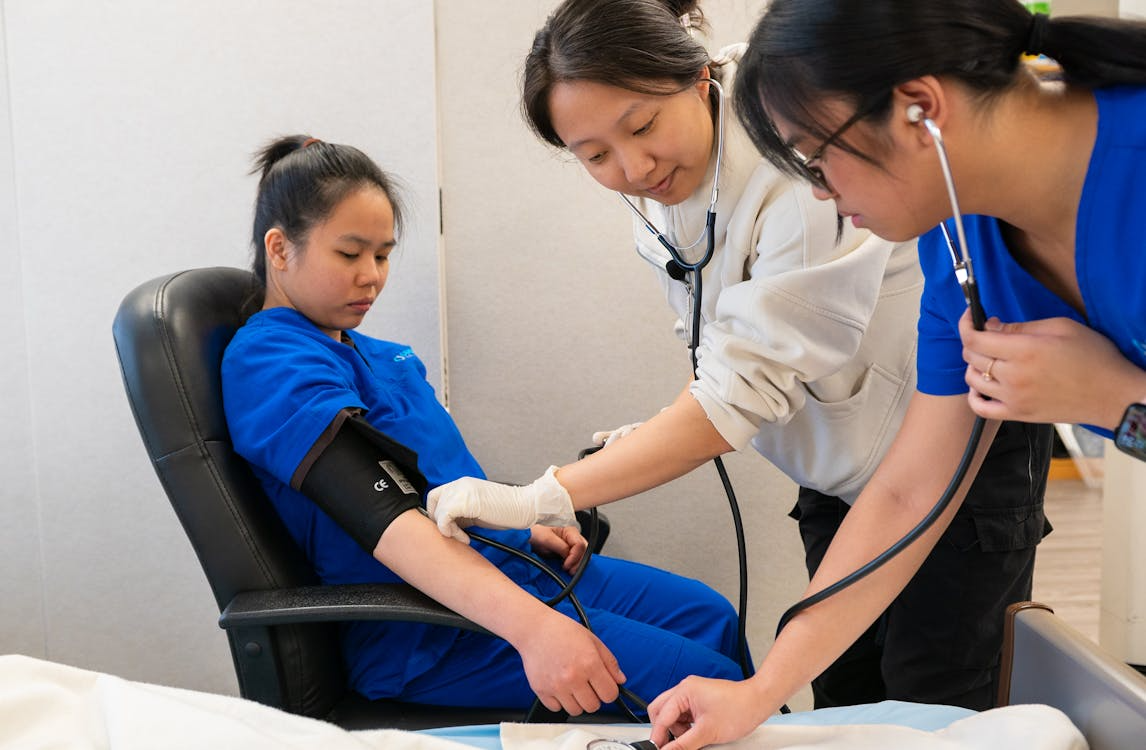
1. Immediate Assessment Steps
The initial response begins with a rapid primary assessment following the ABC framework: Airway, Breathing, Circulation. This evaluation must occur within seconds of reaction identification and determines the severity classification that guides subsequent interventions.
First, assess airway patency, looking for angioedema, stridor, or other signs of obstruction. Next, evaluate breathing effectiveness by noting respiratory rate, oxygen saturation, and work of breathing.
Finally, check circulation by assessing the pulse, blood pressure, capillary refill time, and skin color. This assessment should be performed while simultaneously mobilizing appropriate resources based on the identified severity level.
2. Emergency Equipment Requirements
Every MRI facility must maintain a dedicated crash cart with appropriate emergency medications and equipment that can be rapidly deployed in the event of a contrast reaction. This cart should be positioned in a consistent, accessible location known to all staff members and checked regularly for completeness and expiration dates.
Critical components include airway management supplies (oxygen, bag-valve-mask, oral/nasal airways), intravenous access equipment, and emergency medications including epinephrine, antihistamines, and inhaled beta-agonists. All equipment must be MRI-conditional or clearly labeled with zone restrictions to prevent dangerous projectile incidents during emergencies.
3. Staff Role Assignments
Clear role delineation eliminates confusion during high-stress contrast reactions. Effective management requires, at a minimum, a team leader (typically the radiologist or most senior medical provider), a medication administrator, a documentation specialist, and a resource coordinator who can summon additional help if needed.
Facilities may assign these roles during shift changes or morning huddles, with visual identifiers, such as colored lanyards or badges, helping clarify responsibilities during emergencies. Regular simulation drills reinforce these assignments and identify potential workflow improvements before actual emergencies occur.
4. MRI-Specific Safety Considerations
The MRI environment presents unique challenges during contrast reactions that require specialized protocols. The powerful magnetic field makes traditional crash carts and many medical devices dangerous projectile hazards, necessitating MRI-conditional emergency equipment.
Patient extraction from the bore must be performed without delay while maintaining proper alignment to prevent injury. Communication systems between the scanner room and control area must function effectively despite acoustic isolation. Additionally, access to the patient may be restricted by the scanner configuration, requiring practice in alternative approaches for medication administration and resuscitation efforts that accommodate these spatial limitations.
MRI Contrast Reaction Documentation Requirements

Thorough documentation serves multiple crucial functions beyond the immediate clinical management of contrast reactions. It creates a legal record of the event, facilitates appropriate follow-up care, informs future imaging decisions, and provides data for quality improvement initiatives.
Documentation must begin simultaneously with clinical management, continue throughout the episode, and conclude with clear follow-up instructions and future recommendations.
Critical Elements to Record
Documentation must capture specific details about the contrast agent, including brand name, concentration, total volume administered, route of administration, and injection rate. The patient's vital signs before, during, and after the reaction must be recorded with precise timestamps to establish the reaction timeline.
All clinical manifestations should be described objectively with attention to progression patterns and response to interventions. Every medication administered requires documentation of dose, route, timing, and observed effects. This detailed record becomes essential for future care planning and potential medicolegal review.
Timing Documentation Requirements
Record the exact time of contrast administration, onset of symptoms, each intervention performed, and resolution of symptoms. This chronology allows for evaluation of recognition and response efficiency while establishing the relationship between contrast exposure and clinical manifestations.
Timing elements should capture the progression of the reaction, noting whether symptoms intensified or improved over specific intervals. This helps determine whether the reaction followed typical patterns or presented atypically, informing future risk assessments.
Electronic Medical Record Integration
Modern contrast reaction management requires seamless integration with electronic health record systems to ensure documentation accessibility across care teams. Standardized contrast reaction templates within the EHR facilitate comprehensive documentation while reducing cognitive load during emergencies.
These structured forms should include mandatory fields for contrast details, reaction classification, interventions performed, and follow-up recommendations. The system should automatically generate allergy alerts for future encounters and prompt appropriate management strategies based on reaction severity, such as substituting a contrast agent or premedication when indicated.
Importantly, this documentation must be readily accessible to providers outside the radiology department who may be involved in subsequent care decisions.
Post-Reaction Monitoring Protocol
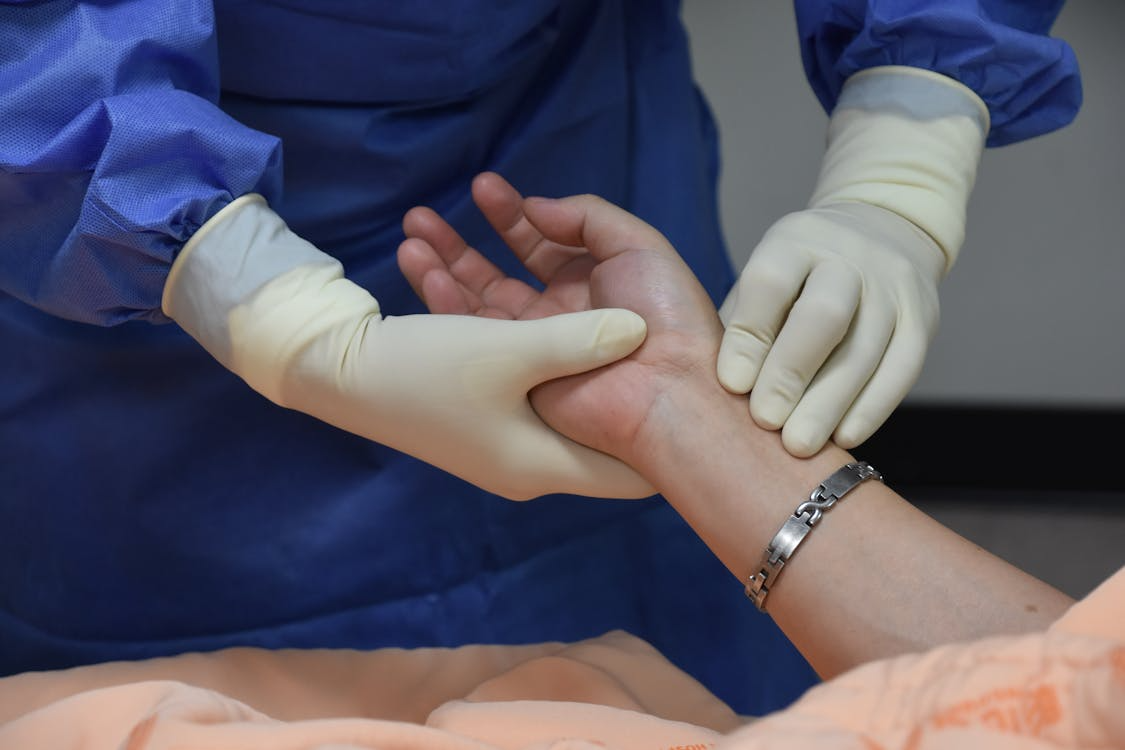
After the acute phase of a contrast reaction, continued monitoring identifies potential biphasic reactions and ensures complete resolution before discharge. Monitoring duration should be proportional to reaction severity:
- Mild reactions: 20–30 minutes of observation after symptom resolution
- Moderate reactions: 1–2 hours with serial vital sign monitoring
- Severe reactions: 4–6 hours minimum; consider hospital admission for patients requiring epinephrine
During observation, assess vital signs at regular intervals, paying close attention to respiratory status and hemodynamic stability. Continuous pulse oximetry provides early warning of respiratory compromise. Patients requiring multiple epinephrine doses, those with cardiovascular comorbidities, or advanced age warrant extended monitoring regardless of apparent resolution.
Discharge Criteria
Before discharge, verify complete symptom resolution with stable vital signs for at least 30 minutes. Confirm the patient has reliable transportation, a responsible adult for monitoring, and the ability to return for care if symptoms recur. Provide written instructions detailing warning signs requiring immediate medical attention. Document the discharge decision with a clear rationale, particularly when protocols are modified based on individual circumstances.
How to Flag Patients for Future Contrast Procedures
Electronic medical record systems should incorporate standardized contrast reaction alerts that appear during order entry, scheduling, and at the point of care. These alerts must specify the contrast agent involved, reaction type, severity, and outcome to guide appropriate management decisions.
Importantly, the system should differentiate between allergic-like reactions, which may warrant agent substitution or premedication, and physiologic reactions that require different management approaches.
Healthcare networks may consider synchronizing reaction documentation across facilities to identify at-risk patients before arrival. This allows appropriate preparation regardless of where patients receive care.
Safe Contrast Administration with ContrastConnect
For imaging centers facing radiologist shortages or seeking to extend coverage hours, maintaining preparedness across every shift presents operational and financial challenges. At ContrastConnect, we address these challenges through a virtual contrast supervision platform that connects your team with experienced radiologists in real time.
Our radiologists supervise more than 55,000 contrast exams monthly and manage 5–10 contrast reactions daily across our partner network. This concentrated experience ensures that when reactions occur at your facility, your team has immediate access to clinicians who routinely navigate contrast emergencies. All contrast supervision follows ACR-aligned protocols and includes standardized, audit-ready documentation to support regulatory compliance and continuity of care.
We integrate seamlessly with your existing workflows through secure, HIPAA-compliant audio and video communication. This model provides the consistent oversight that regulators expect while eliminating the cost and recruitment burden of expanding on-site physician coverage.
Contact us to learn how virtual contrast supervision can strengthen your facility's reaction preparedness and operational efficiency.
Start Your Coverage Assessment Today →
Frequently Asked Questions (FAQs)
What are the side effects of contrast dye MRI?
Side effects of contrast dye used in MRI include nausea, headache, dizziness, warmth at the injection site, or a metallic taste. These are generally mild and short-lived. Less commonly, patients may experience hives or itching.
Severe allergic-like reactions are rare with MRI contrast, but can involve breathing difficulty or facial swelling. In people with severe kidney disease, there is a very small risk of nephrogenic systemic fibrosis, so kidney function is often checked beforehand. These considerations help clinicians assess safety before administering contrast.
Should we document mild MRI contrast reactions that resolve without intervention?
Yes. All contrast reactions require documentation regardless of severity. Even mild reactions provide valuable information for future imaging decisions and support quality improvement initiatives. Thorough documentation also demonstrates appropriate monitoring and protects against potential liability.
Are there regulatory requirements for documenting contrast reactions?
The Joint Commission, CMS, and ACR accreditation programs all require documentation of contrast reactions, including standardized forms and reporting mechanisms. State regulations may impose additional requirements. Beyond compliance, documentation enables analysis of reaction patterns and evaluation of prevention strategies.
How does ContrastConnect ensure safe contrast supervision?
ContrastConnect provides real-time virtual contrast supervision through a secure, HIPAA-compliant platform, with radiologists immediately available to guide reaction management and ensure proper documentation.
Our team supervises over 55,000 contrast exams monthly and manages 5–10 contrast reactions daily, bringing unmatched clinical experience. Facilities partnering with ContrastConnect benefit from audit-ready documentation and the ability to extend coverage without adding on-site radiologists.
*Note: Information provided is for general guidance only and does not constitute medical, legal, or financial advice. Pricing estimates and regulatory requirements are current at the time of writing and subject to change. For personalized consultation on imaging center operations and virtual contrast supervision, contact ContrastConnect.
Trusted Nationwide
















































74,000+
Contrast exams supervised monthly
64,000+
Hours of supervision monthly
2,700+
Technologists certified
0s
Of imaging partners nationwide
30+
Contrast reactions treated monthly
0%
Requested hours covered
Connect with us.


